


 More...
More...



 More ..
More ..


When you see the VeriGMP® icon on a product, please know that the product is manufactured in a facility that is both AUDITED and VERIFIED to be GMP compliant. Participating brands and products are listed here.
VeriGMP guarantees that listed brands and products are manufactured in a NSF certified factory.


For those of us who read supplement labels, we have all seen the typical GMP icons that look fancy but don’t mean a whole lot. Like these “designer icons”.



The above icons carry no guarantees of any compliance with GMP protocols! These are simply the opinions of the brand owner, who designs the labels. It’s advertising.
We estimate that about 80% of all products that claim “GMP Certification” are actually not manufactured in GMP certified factories. The two most common GMP certification organizations are NSF and UL. Neither of them allow brands to display their logo on their products even if they are made in GMP certified manufacturing facilities unless in some rare cases the brands can pony up tens of thousands of dollars per each production lot.
This is why brand owners don’t have such an enormous incentive to contract with factories that go through with the enormous costs and efforts to maintain a GMP certification, so half of brand owners willingly choose factories that are not GMP certified – for a lower price of course.
Many other brand owners get tricked into thinking they are contracting with GMP certified factories.
Unfortunately, NSF unwittingly made it easier for factories to bluff brand owners by issuing identical GMP certifications for the following 3 operations (a) Manufacturing Facility (b) Warehouse Facility and (c) Distribution Facility.
This has caused lots of factories to obtain the inferior GMP certifications of operating a warehouse facility and pretend to brand owners to be GMP certified as manufacturers.
For those brand owners that are honestly trying to hire properly certified GMP certified manufacturers our sister company PureNSM offers a guide that anyone can download here: Pitfalls to Avoid and Questions to Ask Supplement Manufacturers (before you hire them)
If you operate a brand selling on Amazon, it’s most important to manufacture your supplements in a GMP certified facility by UL and/or NSF.
Amazon is cracking down on supplements and even experienced sellers are having a hard time. Amazon’s requirements change often, the most current ones as of January, 2023 state that sellers must have the following:
However, apart from that, it’s prudent for other reasons: purity and potency.
You don’t boil your supplements, do you? You don’t taste the capsules and tablets you swallow either. This is why it’s more important for factories that manufacture those items to follow GMP protocols.
Microbiological testing has to be available from a BLS-2 micro lab using plate methods and positive control pathogens and not only rapid microbiological testing devices such as Solaris.
Furthermore many herbs are derived from roots that grow in countries that until recently did not regulate against leaded gasoline. This has caused the herbs to find their way into products sold worldwide to be contaminated by lead. Finally when you purchase herbal supplements you want them to be “for real”, meaning you want what is stated on the label to be what is actually in the product. Manufacturing is a complicated business. Please let me explain the pitfalls that can only be avoided if the supplements are made in a GMP certified facility.
When you see the VeriGMP icon on your supplements, rest assured that 100% of all incoming botanical raw materials have been tested for identity using the most advanced ID testing methods available, abbreviated HPTLC. Not to be confused with another system used to analyze for potency called HPLC.
We estimate that about 30% of botanical raw materials sold to manufacturers are adulterated. We know that the only way to protect the consumer from those adulterated herbal raw materials is to test them at the point of manufacture for identity and authenticity. For the inquisitive: HPTLC stands for High-performance thin-layer chromatography. HPLC stands for High-performance liquid chromatography.
Make sure your contract manufacturer uses HPTLC system from CaMag and has hundreds of methods developed. HPTLC is considered by FDA to be better than DNA analysis because it does not rely on fragments of DNA that is frequently damaged when plants have been ground to powder. Make sure your manufacturer tests for the following for every single botanical in your product. The best instrument for this is the HPTLC from CaMag:


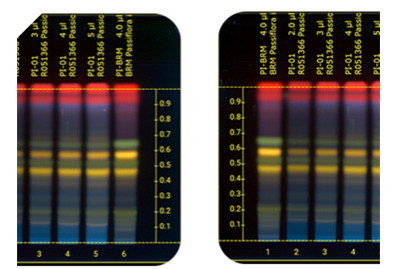


Unfortunately the regulations governing raw material vendors for supplements are subject to a more relaxed (less stringent) regulation called 21 CFR 117 and not 21 CFR 111, which are stricter and govern supplement manufacturing facilities. FDA claims the 21 CFR 117 regulation needs to be less strict because they sell to several different industries, not only the supplement industry, such as foods, cosmetics, and pharmaceuticals. This shifts the burden of quality control of raw materials to the supplement manufacturers. This is exactly why it’s so important that they adhere to strict GMP protocols.
You don’t wash or boil most supplements; in fact, you swallow most whole. That’s precisely why it’s so important to only ingest trustworthy supplements. VeriGMP makes sure your supplements have passed the purity test with a battery of microbiological analytical methods.
Make sure your manufacturer uses the Soleris rapid microbiology systems, the traditional plate count methods in a BSL-2 laboratory and the 3M parafilm plate reader. It’s not possible to rely on one system alone and that is why we use all three.

Heavy metals are not fun, they are found in food but also in supplements, especially supplements from roots. Heavy metals such as lead find their ways to plant materials from decades of use of leaded gasoline in India and other countries. Make sure to calculate your levels of lead because you can be liable for large fines based on California Proposition 65, even if you don’t have a business in California.
It’s important to analyze both raw materials and “finished-goods” for quality (identity, potency, purity). Make sure your manufacturer tests for potency twice, once when they receive the raw materials and once when the product has been manufactured, at the end stage of manufacturing. This is the only way to make sure what is stated on the label is actually in the supplements. This is done with a variety of analytical instruments, HPLC being one of them.
Potency has a lot to do with overages, blend uniformity, particle size of ingredients, degradation of ingredients over time and the interaction of different ingredients with each other. It can become more complicated than expected because you are blending many tons of materials with the goal of getting a perfect blend into a capsule that can be about 250mg to 1000 mg.


